Impact Factor:5.518
DOI number:10.1016/j.colsurfa.2023.131262
Affiliation of Author(s):中南大学
Journal:Colloids and Surfaces A: Physicochemical and Engineering Aspects
Key Words:Cassiterite; Calcite; Flotation; Cinnamohydroxamic acid; Pb2+; Activation.
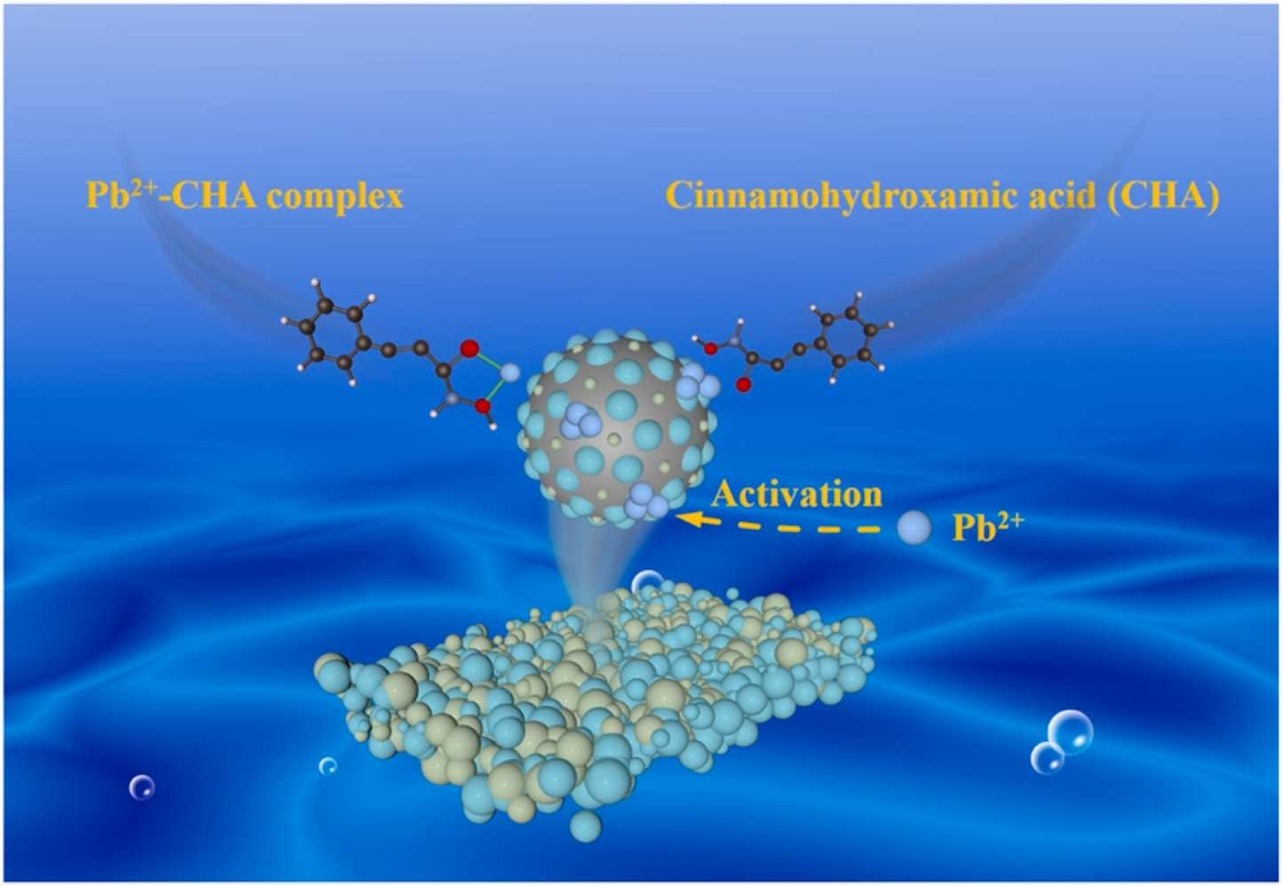
Abstract:This study employs cinnamohydroxamic acid (CHA) as the collector in the flotation separation of cassiterite/calcite. Pb2+ ion is used as the activator to improve the collecting ability of CHA. This reagent system consisting of CHA collector and Pb2+ activator can achieve a higher recovery of cassiterite of ∼90% than that of calcite of ∼30%. A greater increase of 27.74% in C atomic concentration on the Pb2+-treated cassiterite surface than that of 15.21% on the Pb2+-treated calcite surface after the treatment with CHA indicates a higher adsorption amount of CHA on the Pb2+-treated cassiterite surface than that on the Pb2+-treated calcite surface, which results in its stronger ability to collect the Pb2+-activated cassiterite particles compared to the Pb2+-activated calcite particles. The treatment with CHA results in a smaller shift of ∼20 mV in the zeta potential of the Pb2+-treated cassiterite particles than that of ∼45 mV in the zeta potential of the Pb2+-treated calcite particles, which is in contradiction with the results of the X-ray photoelectron spectroscopic tests. A novel activation mechanism of Pb2+ ion for CHA flotation of cassiterite is proposed to account for this paradoxical observation. The formation of the Pb-CHA complexes is this activation mechanism of Pb2+ ion.
Indexed by:Journal paper
Correspondence Author:田孟杰
Discipline:Engineering
First-Level Discipline:Mining Industrial Engineering
Document Type:J
Volume:666
Translation or Not:yes
Included Journals:SCI