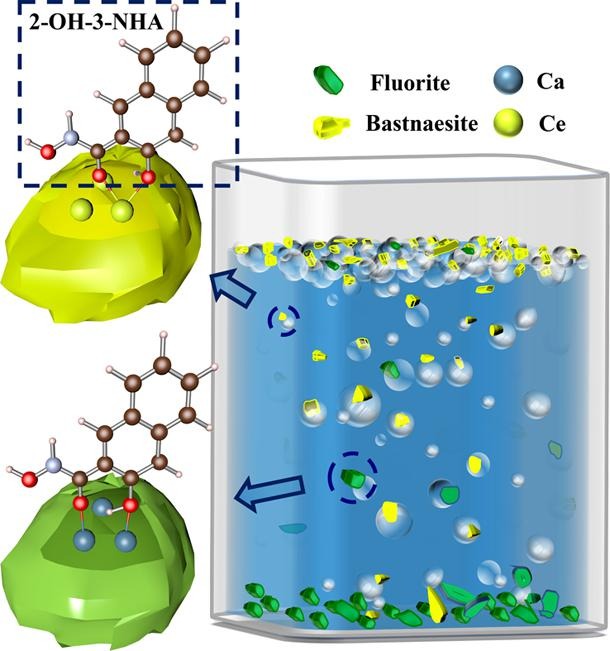
Interaction mechanism of 2-hydroxy-3-naphthyl hydroxamic acid and 1-hydroxy-2-naphthyl hydroxamic acid in the flotation separation of bastnaesite/fluorite: Experiments and first-principles calculations
发布时间:2022-04-10
点击次数:
影响因子:7.312
DOI码:10.1016/j.seppur.2021.120307
发表刊物:Separation and Purification Technology
刊物所在地:England
关键字:Bastnaesite; Fluorite; Flotation; Collector; Naphthyl hydroxamic acid
摘要:Bastnaesite ((Ce, La, Pr, Rb)CO3F) is an important source of light rare earth elements and is commonly associated with calcium (Ca)-containing minerals such as fluorite (CaF2), calcite (CaCO3) and dolomite ((Ca, Mg)(CO3)2). Froth flotation is an efficient method to separate bastnaesite and Ca-containing minerals. 2-hydroxy-3-naphthyl hydroxamic acid (2-OH-3-NHA) and 1-hydroxy-2-naphthyl hydroxamic acid (1-OH-2-NHA) are two widely used flotation collectors for bastnaesite and this manuscript investigates their performance and interaction mechanism in the flotation separation of bastnaesite/fluorite. The development of novel collectors must be based on the investigations of this manuscript. The flotation experimental results show the excellent selectivities of NHAs and the better selectivity of 1-OH-2-NHA than 2-OH-3-NHA. Zeta potential measurements suggest stronger adsorptions of NHAs on the bastnaesite surface than fluorite, which results in the excellent selectivities of NHAs. First-principles calculations demonstrate that naphthalene ring-bonded OH group of NHAs shows non-selective adsorption towards the surfaces of bastnaesite and fluorite. The better selectivity of 1-OH-2-NHA than 2-OH-3-NHA is explained by the lower reactivity of naphthalene ring-bonded OH group due to the stronger steric hindrance effect of naphthyl group towards this OH group.
论文类型:期刊论文
通讯作者:田孟杰
学科门类:工学
一级学科:矿业工程
卷号:285
页面范围:120307
是否译文:否
发表时间:2022-03-15
收录刊物:SCI
发布期刊链接:https://www.sciencedirect.com/science/article/pii/S1383586621020116
附件: