DOI number:10.1016/j.colsurfa.2021.127600
Affiliation of Author(s):中南大学
Journal:Colloids and Surfaces A: Physicochemical and Engineering Aspects
Key Words:Adsorption; GCMC; N-O co-doped; Porous carbon; Toluene
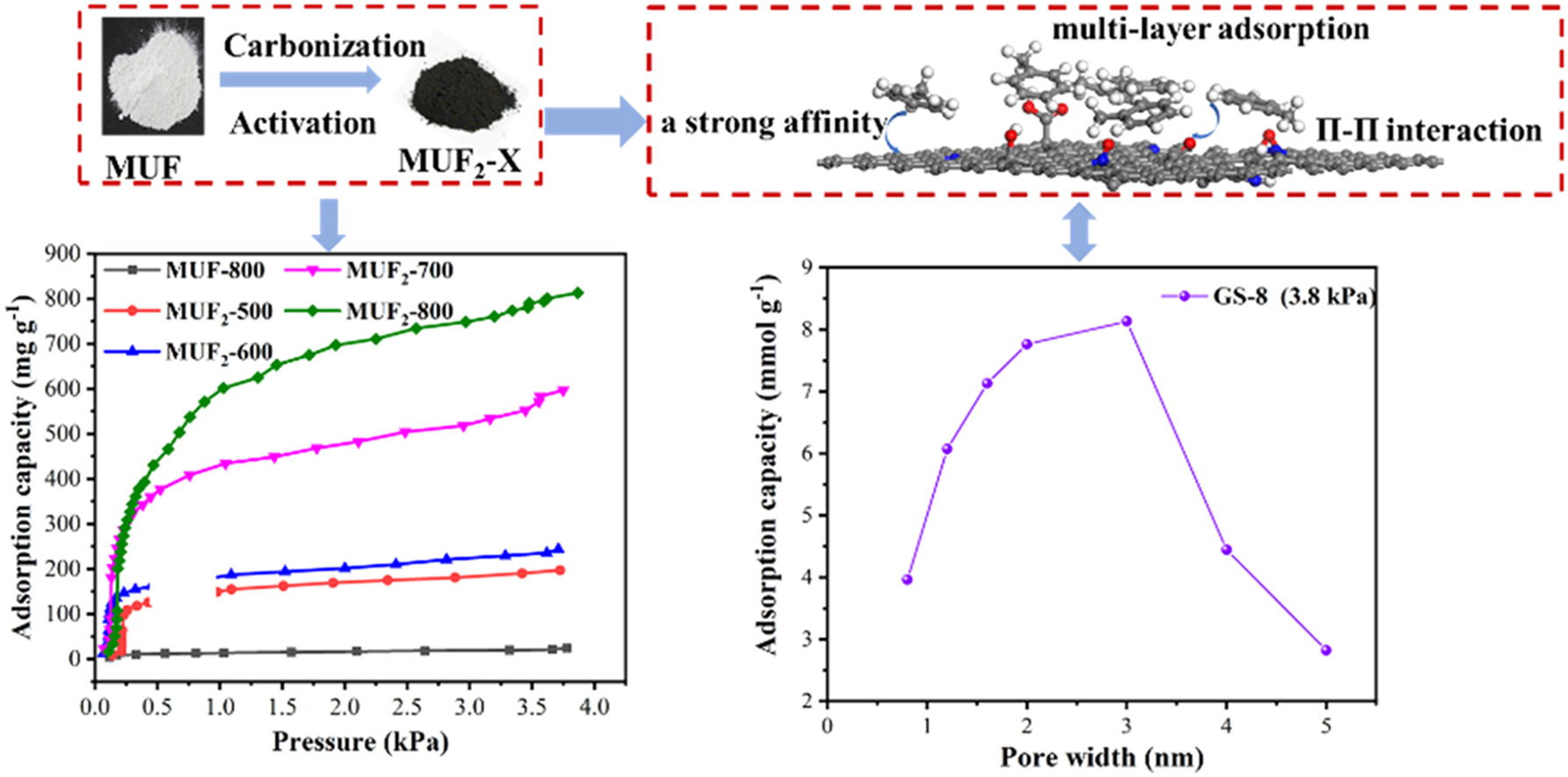
Abstract:The N-O co-doped porous carbons with high specific surface areas and hierarchical pore structures were synthesized by KOH activation method using melamine urea-formaldehyde resin as the carbon precursor. The adsorption properties of toluene on all the samples were studied by experiments, density functional theory (DFT) calculation and grand canonical Monte Carlo (GCMC) simulation. Results showed N-O co-doped porous carbon exhibited a great specific surface area (2784.53 m2 g−1), a desirable pore volume (1.83 cm3 g−1), a high nitrogen (16.16%) and oxygen content (15.75%), and especially an excellent toluene adsorption performance (813.6 mg g−1, 25 °C). By correlating the adsorption capacity with physical and chemical property parameters, the main factors affecting the toluene adsorption were pore size and specific surface area. Furthermore, according to the theory calculation, the interaction between toluene and toluene can be improved by the N-O functional group and the multilayer adsorption can be formed. Considering this, we concluded that the optimal adsorption pore size of N-O co-doped porous carbons was 3–7 times as much as the toluene dynamic diameter. Such optimal adsorption pores not only provided a pathway and adsorption sites for toluene, but also had higher adsorption capacity of toluene. This study can be used to promote the molecular design of adsorbent of heteroatomic doping with an optimal adsorption pore size.
Indexed by:Journal paper
Document Code:127600
Volume:632
ISSN No.:09277757
Translation or Not:no
Date of Publication:2022-01-02
Included Journals:SCI
Links to published journals:https://www.sciencedirect.com/science/article/pii/S0927775721014692?via%3Dihub