DOI number:10.1021/acs.energyfuels.1c02313
Affiliation of Author(s):中南大学
Journal:Energy and Fuels
Key Words:Adsorption, Alkali metals, Carbon, Doping, Selectivity
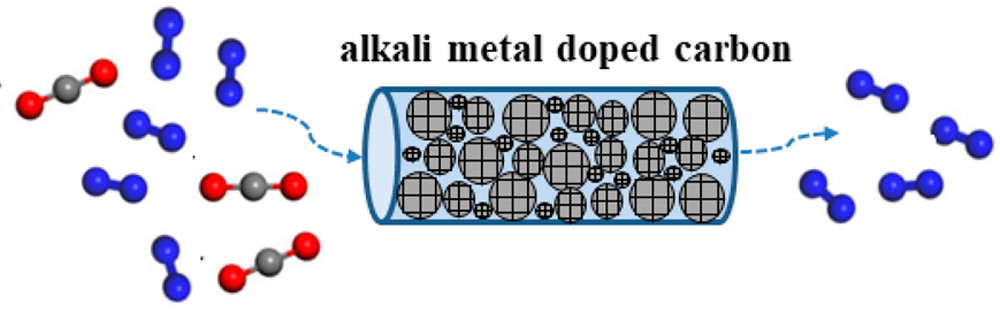
Abstract:Developing an adsorbent with high adsorption capacity and high selectivity is of great importance for the post-combustion CO2capture. Inspired by the theoretical calculation findings that the introduction of alkali metal atoms (e.g., Li, Na, and K) into the carbon framework can significantly increase the CO2adsorption capacity through the enhanced electrostatic interaction, herein, we have successfully developed a series of alkali metal doped carbons through a simple and effective strategy. The systematic characterization results prove that alkali metals are uniformly introduced into the carbon framework without changing the pore size distribution and amorphous structure of the original material, but slightly reducing the specific surface area. The adsorption performances from the static adsorption and the dynamic breakthrough experiments with a binary mixture of CO2/N2(15/85, v/v) reveal that the doping of alkali metals can greatly enhance the capture and separation of CO2, in which the lithium-doped porous carbon presents the best CO2uptake of 4.1 mmol/g at 25 °C (48.3% higher than that of undoped carbon) with the highest CO2/N2selectivity of 47 and fastest adsorption rate. The high adsorption capacity and selectivity presented are comparable to the data reported in the previous literature. The density functional theory and grand canonical Monte Carlo molecular simulation results demonstrate that, compared with N2, alkali metal doped carbon has a stronger binding energy and higher adsorption density for CO2and thus increases the CO2/N2selectivity to a greater extent. This proof-of-concept work paves an alternative way for the development of high-performance CO2adsorbents.
Indexed by:Journal paper
Volume:35
Issue:19
Page Number:15962 - 15968
ISSN No.:08870624
Translation or Not:no
Date of Publication:2021-10-07
Included Journals:SCI
Links to published journals:https://pubs.acs.org/doi/10.1021/acs.energyfuels.1c02313