DOI number:10.1016/j.apsusc.2025.164502
Journal:Applied Surface Science
Key Words:Adsorption energy; Density functional theory; Density of states; High-entropy alloy; Oxygen evolution reaction
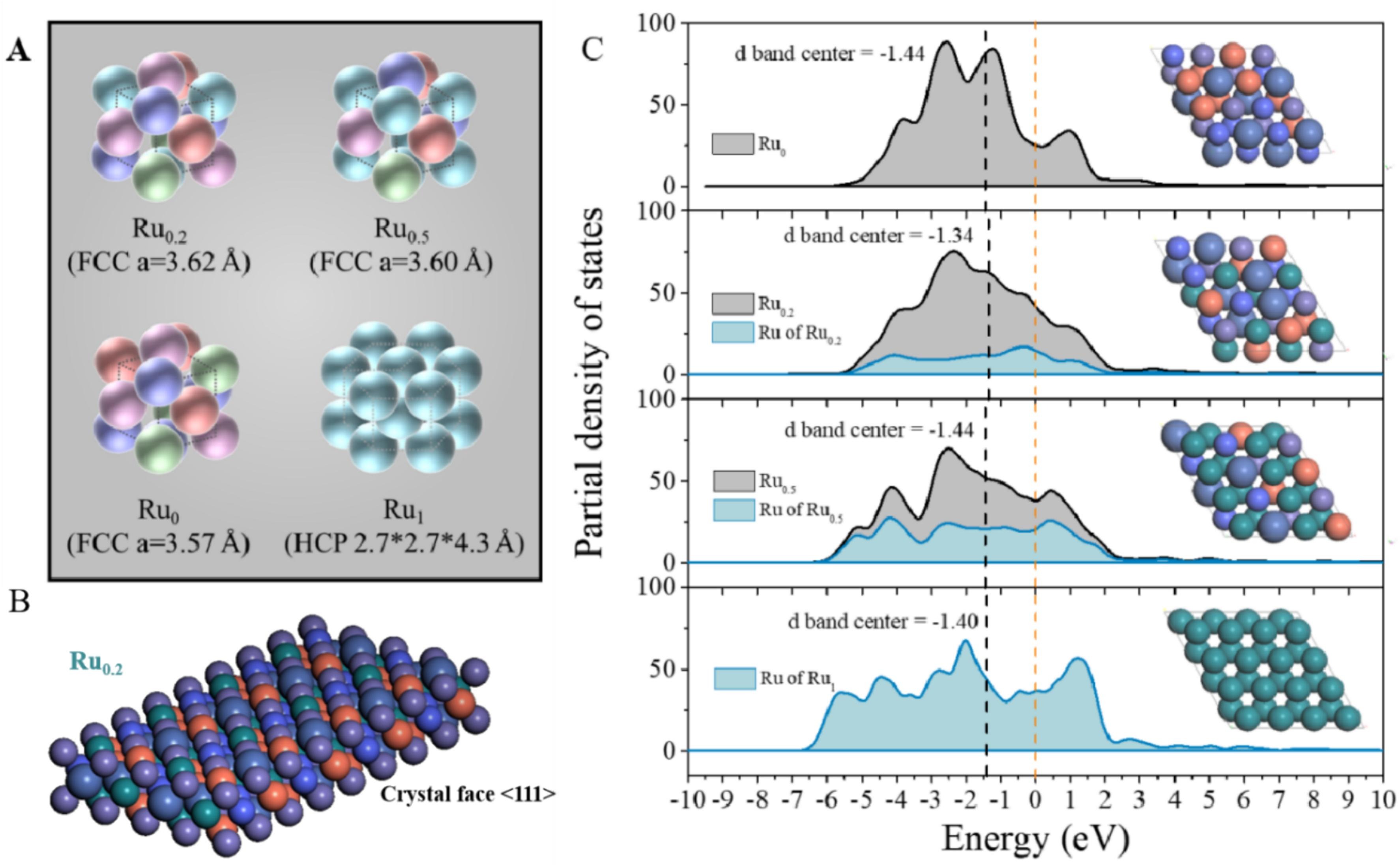
Abstract:The expensive material costs of precious metals long limit their use as catalysts. The development of High-Entropy Alloy (HEA) materials provides a feasible approach to combine high performance and lower cost. Therefore, Ru-containing HEA nanoparticles are synthesized on carbon nanofibers. Among them, Ru0.2@CNFs reveals the best catalytic oxygen evolution reaction (OER) performance and extraordinary stability for the 1000th cycle. Moreover, the Ru-containing multimetallic catalyst models are calculated based on crystal information obtained from X-ray diffraction result. The great electrocatalytic potential of the Ru0.2 <1 1 1> crystal face is indicated by the density of states and d-band center, which might benefit from the stronger atomic interaction caused by high-entropy mixing. The largest configuration entropy of Ru0.2 HEA (1.58 R) gives a reasonable explanation for its remarkable stability in OER electrocatalysis. In addition, the similar adsorption energies of *OH demonstrate the abundant active sites on the surface of RuFeCoNiCu HEA. As a result, the high-entropy material Ru0.2@CNFs can provide potential nanocatalysts with reduced noble metal loading for practical applications. Besides, significant insights for performance prediction in other high-entropy materials are given by the modeling and calculations of HEAs.
Indexed by:Journal paper
Document Code:164502
Volume:715
Translation or Not:no
Date of Publication:2025-08-31
Included Journals:SCI
Links to published journals:https://www.sciencedirect.com/science/article/pii/S0169433225022184?via%3Dihub