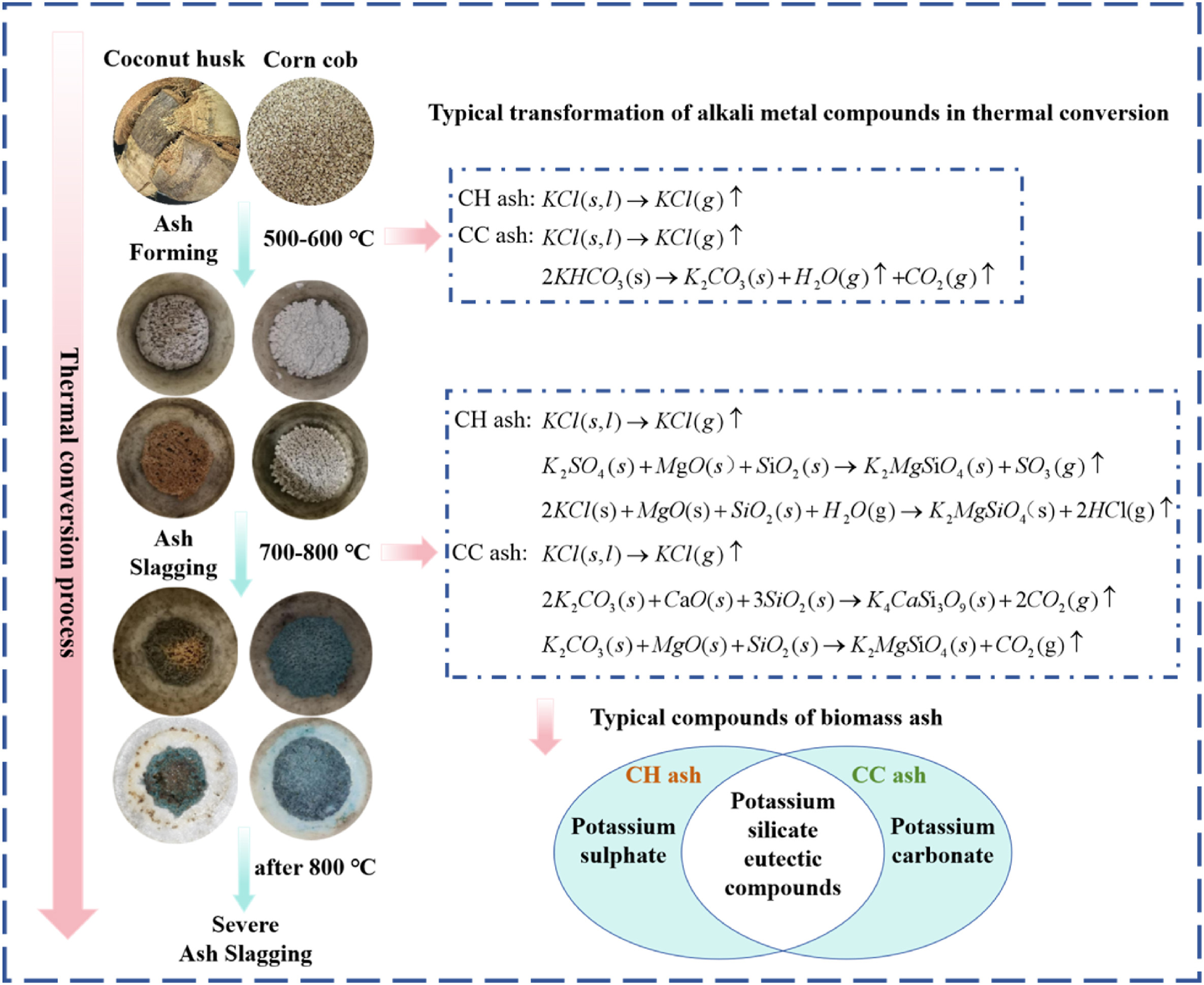
Mechanistic insights into alkali metal migration and slagging behavior in K-type biomass ash during thermal conversion
发布时间:2025-06-14
点击次数:
DOI码:10.1016/j.energy.2025.136800
发表刊物:Energy
关键字:Alkali metal migration; Ash slagging; Biomass ash; Thermal conversion
摘要:Severe slagging phenomena in biomass ash under elevated temperatures have been associated with alkali metal migration. It is imperative to conduct research on alkali metal migration in biomass ash slagging during thermal conversion. Here, the migration of alkali metals in two K-type biomass ashes (coconut husk ash and corn cob ash) during thermal conversion was investigated. The study concluded that alkali metal migration is a primary cause of biomass ash slagging, with elevated ashing temperatures promoting this migration. At temperatures above 700 °C, silicate eutectic compounds formed in biomass ash, promoting intensified ash slagging. KCl, K2SO4, and K2CO3 all exhibit a tendency to transform into eutectic silicate compounds in high-temperature environments. The water leaching treatment of biomass raw materials notably inhibited the formation of ash, and the ash yield decreased by 55.58 %. This research explores alkali metal migration in K-type biomass ash during thermal conversion and explores its impact on biomass ash slagging. This mechanistic understanding advances predictive capabilities for biomass combustion systems and informs the development of anti-slagging technologies in thermochemical conversion applications.
论文类型:期刊论文
论文编号:136800
卷号:329
是否译文:否
发表时间:2025-06-14
收录刊物:SCI
发布期刊链接:https://www.sciencedirect.com/science/article/pii/S0360544225024429?via%3Dihub
上一条: Three-Dimensional Bioprinted Scaffolds Loaded with Multifunctional Magnesium-Based Metal–Organic Frameworks Improve the Senescence Microenvironment Prompting Aged Bone Defect Repair
下一条: Solvent-free one-step simple synthesis of N, O-doped microporous carbon using K2CO3 as an activation agent and their application to CO2 capture: Synergistic effect of pore structure and nitrogen–oxygen functional groups