DOI number:10.1016/j.apsusc.2024.160769
Affiliation of Author(s):中南大学
Journal:Applied Surface Science
Key Words:Manganese dioxide; Oxygen vacancy; Ozone removal; Potassium ion; Water resistance
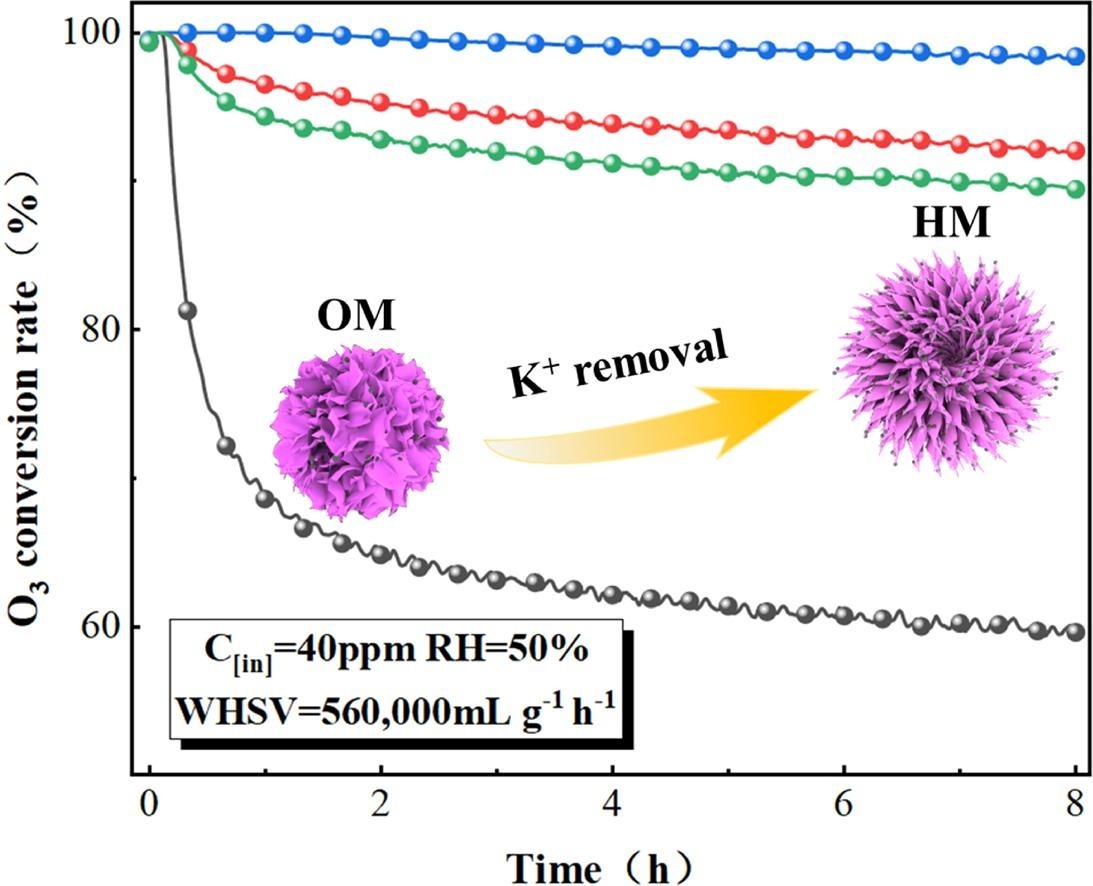
Abstract:Near ground level and in indoor environments, ozone is extremely hazardous, and its removal under harsh conditions (high humidity and high air speed) poses a significant challenge. In this work, a series of catalysts for ozone decomposition were prepared by simple redox reactions and acid treatment under normal pressure and room temperature. The sample H20M-3 (1:20 solid–liquid mass ratio and 3 h stirring time) exhibited excellent ozone decomposition and water resistance properties. Ozone conversion efficiency exceeded 99 % in 8 h at 40 ppm ozone, a temperature of 30 °C, 50 % relative humidity, and a weight hourly space velocity (WHSV) of 560,000 mL g-1h−1. Even under harsh conditions of 90 % relative humidity, the conversion efficiency remained at 70 % after 8 h. We investigated the effects of acid treatment on the catalysts and found that potassium ion decrease resulted in the formation of nanoflower-like structures and enhanced the mobility of oxygen species, accelerating ozone decomposition. Density functional theory (DFT) calculations indicated that the potassium ion decrease contributed to the formation of oxygen vacancies and enhanced the water resistance of the catalysts. Moreover, we revealed the decomposition mechanism of ozone when coexisting with water molecules, which provided new insights for related studies.
Indexed by:Journal paper
Document Code:160769
Volume:671
Translation or Not:no
Date of Publication:2024-09-29
Included Journals:SCI
Links to published journals:https://www.sciencedirect.com/science/article/pii/S016943322401482X?pes=vor