DOI number:10.1016/j.ces.2025.121615
Journal:Chemical Engineering Science
Key Words:CO2 adsorption; GCMC&DFT; N, O-doped microporous carbon materials; Nitrogen-oxygen functional groups; One-step synthesis; Sugarcane bagasse
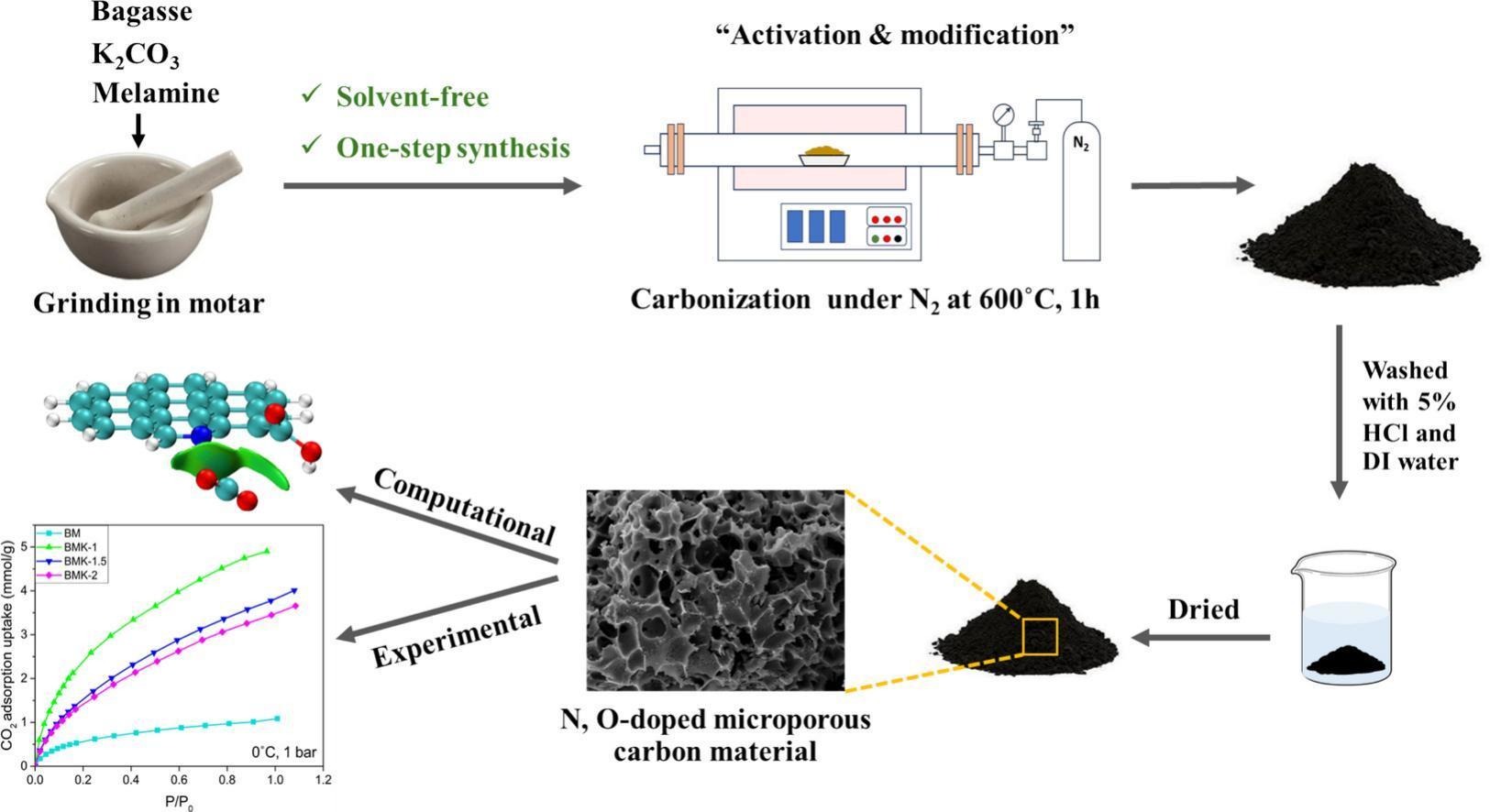
Abstract:Preparing N, O-doped microporous carbons requires a multi-step process, which is expensive and time-consuming. The preparation methods presented in this work are easy, solvent-free, and more sustainable, using sugarcane bagasse as a carbon precursor, melamine as a nitrogen source, potassium carbonate as an activator, and carbonization at a low temperature. The prepared carbons (BMK-1) showed the highest CO2 adsorption performance (3.24 mmol/g at 25°C and 4.90 mmol/g at 0°C, 1 bar). An in-depth study was performed to analyze the influence of narrow micropores with different pore ranges and nitrogen–oxygen atoms doping on CO2 adsorption performance. GCMC simulations and weak interaction analyses showed that a pore size of approximately 0.7 nm is suitable for CO2 adsorption. The existence of nitrogen–oxygen groups enhanced the Van der Waal interaction between samples and CO2 molecules. It showed that the pore structure and nitrogen–oxygen group are synergistic factors contributing to CO2 adsorption properties.
Indexed by:Journal paper
Document Code:121615
Volume:311
Translation or Not:no
Date of Publication:2025-06-01
Included Journals:SCI
Links to published journals:https://www.sciencedirect.com/science/article/pii/S0009250925004385?via%3Dihub